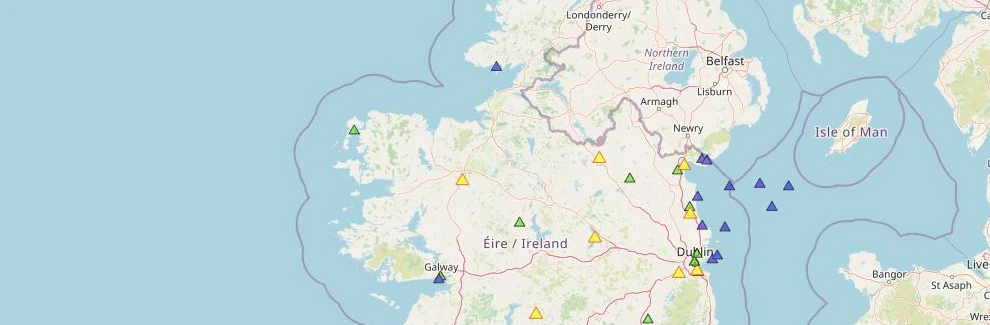
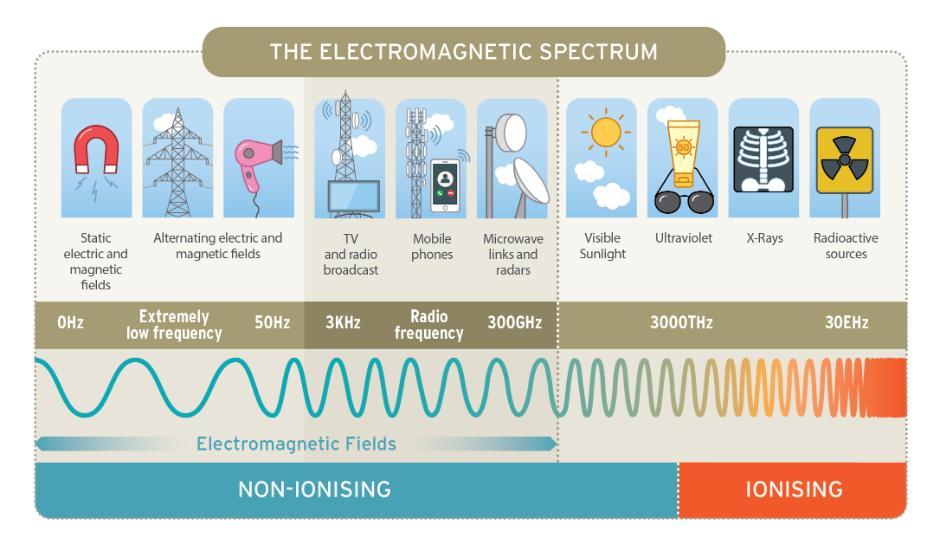
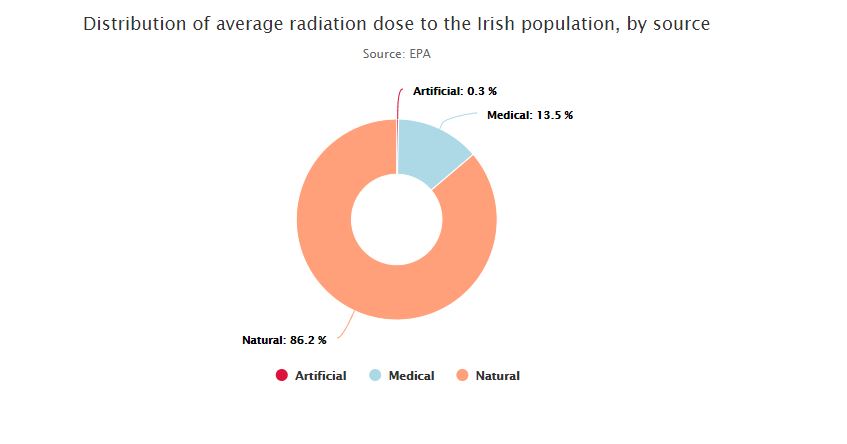
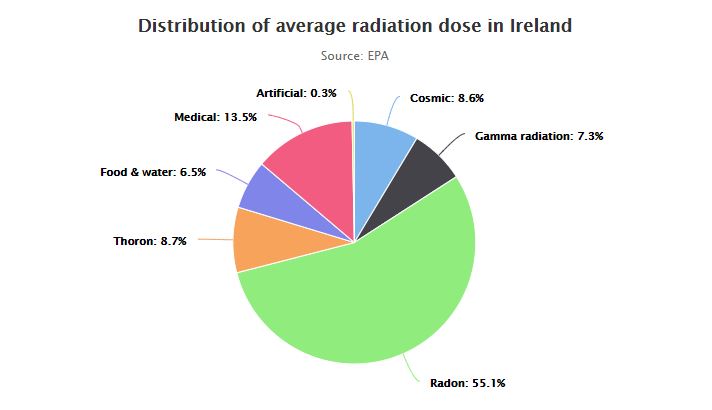
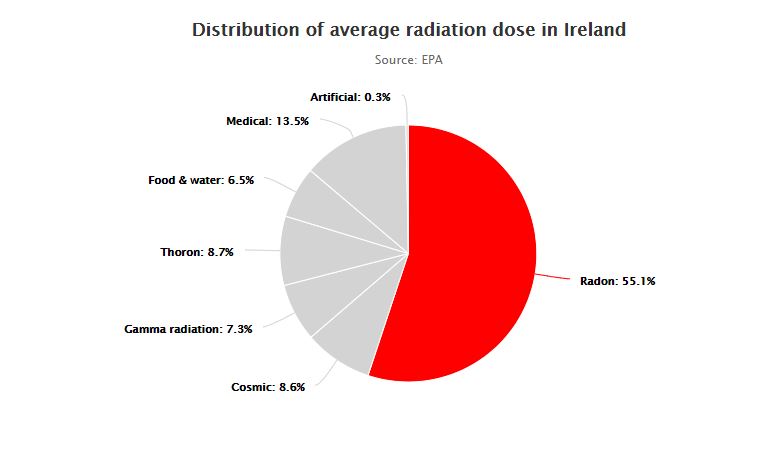
On one end of the spectrum, known as ionising radiation, the radiation has enough energy to cause damage to human cells and can potentially lead to cancer. This can come from man-made radioactive waste, X-rays, nuclear accidents or it can come from naturally occurring sources such as radon, radiation in food and soils or indeed radiation from outer space.
On the other end of the spectrum, known as non-ionising radiation, radiation does not have enough energy to break up molecules and no health effects have been identified for members of the public below guideline levels. This type of radiation can come from man-made mobile phones, electrical appliances, power lines, microwave ovens or it can come from natural sources such as the earth’s magnetic field, lightning storms, the sun or even our own bodies. This type of non-ionising radiation is often called Electromagnetic fields (EMF).